Unleaded Gasoline
#1
Unleaded Gasoline
The 69' Cutlass was born to burn leaded fuel. 35 years later that's not available at the pump where I live (No where). Getting the car running after sitting for decades has its issues...bad connections, dead rubber, fuel tank full of spoooge, and so on. Fired up the Rocket 350 and runs pretty good considering. Whats the unleaded fuel going to do to the engine, valves, ect until I can tear into the engine ?
H
H
#4
The Premium pump (91 oct) at my local fuel stop does not say ethanol added, but the unleaded and mid grade are labeled 10% ethanol added. Does anyone know if they dont add ethanol to the "high test" here in The Rocky Mtns of Colorado. Elevation 6000' or anywhere else for that matter ?
#5
Unleaded fuel should be fine, as long as the octane rating is sufficient and you're not constantly towing or racing.
Ethanol reduces the amount of available energy in a given volume of fuel, so in a carbureted engine, gasoline with ethanol will run just a bit leaner than it should.
Ethanol may also degrade rubber fuel system parts.
- Eric
#7
Pretty sure all Colorado stations are a blended fuels
There are like two stations in the Denver area that sell Ethanol free pure gas or clear gas I think it is called. Here is a link for Ethanol-free stations in Colorado (and other states too) http://pure-gas.org/?stateprov=CO
#8
Replace your rubber fuel lines with todays fuel line. Put a good filter in your carb or use a quality inline one. Clean your tank out really well and rebuild your carb with a new kit. The ethanol has its issues but these old cars will run fine on it as long as you perform a tuneup accordingly. Your altitude may give you some problems, but there are a lot of cars and trucks running up there. Remember posted tuneup specs are a guide to get you close, it's up to you to tweak it to what the engine wants.
#9
X10 on using ethanol FREE gas with any equipment that wasn't originally designed to use that crap.
I run "real" (as it can get) gas in all my old school engines and small lawn n garden equipment as well. Ethanol will rot any fuel system not designed to handle it...even the systems designed to handle it struggle. The race car gets a sip of Sunoco Supreme 112...uuummm! Sunoco 112 has lead yeay!
If it makes you feel better you can add some additives such as 104 octane boost and an occasional isopropal dry gas as well as some led additive. But its really not necessary as long as you don't have any driveability problems. Use the blue marine stabil for storage with a full tank of course. Im trying out the new stabil for metal tanks in two of my old cars. So no reports on that yet.
Tetraethyllead, "Leaded gas" was on its way out in the early 70s. About 30 years ago, 1986 it was pretty much gone. It was down right not available to the general public about 20 years ago, 1995-96ish if memory serves me.
I run "real" (as it can get) gas in all my old school engines and small lawn n garden equipment as well. Ethanol will rot any fuel system not designed to handle it...even the systems designed to handle it struggle. The race car gets a sip of Sunoco Supreme 112...uuummm! Sunoco 112 has lead yeay!
If it makes you feel better you can add some additives such as 104 octane boost and an occasional isopropal dry gas as well as some led additive. But its really not necessary as long as you don't have any driveability problems. Use the blue marine stabil for storage with a full tank of course. Im trying out the new stabil for metal tanks in two of my old cars. So no reports on that yet.
Tetraethyllead, "Leaded gas" was on its way out in the early 70s. About 30 years ago, 1986 it was pretty much gone. It was down right not available to the general public about 20 years ago, 1995-96ish if memory serves me.
#10
There are like two stations in the Denver area that sell Ethanol free pure gas or clear gas I think it is called. Here is a link for Ethanol-free stations in Colorado (and other states too) http://pure-gas.org/?stateprov=CO

#11
Here is a website you can go to and find stations selling ethanol free gasoline. I used it on a trip to Louisiana last year and it worked.
http://pure-gas.org/index.jsp?stateprov=TX
http://pure-gas.org/index.jsp?stateprov=TX
#12
I try to put Shell fuel in my Olds powered car and truck. The occasional tank of the ethanol blend is okay in the Olds powered 4x4 but it is EFI and and probably helps with freeze up, considering it runs in -40C.
#13
since ethanol is an alcohol and subject to holding water moisture i always keep some dry gas in the tank and even with old fuel lines and fuel pump diaphragms the dry gas along with proper octane for everyday driving should keep you motoring along
#14
Ethanol (booze), Isopropanol (rubbing alcohol, and, usually "Dry Gas"), and Methanol (wood alcohol) are ALL alcohols.
ALL of them have a polar hydroxyl (OH) group on one end and a non-polar hydrocarbon (CHHH) group or groups on the other end, so all will dissolve in BOTH oil-based (nonpolar) and water-based (polar) solvents.
Methanol is the simplest: Methane with an OH group, or

Ethanol is the next-simplest: Ethane with an OH group, or

Isopropanol has 3 carbons: A propane with an OH on the middle molecule, or,
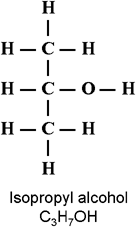
Each of these molecules has a slightly different affinity for water and for gasoline, but will dissolve in both, and, by doing that, will get both to mix together, which would not otherwise occur.
You use "Dry Gas" when you have water in your gasoline in order to get the water to mix with the gas, instead of sloshing around like a big bubble at the bottom of your tank or float bowl, and being fed to the engine, where it won't burn. The "Dry Gas" (or "alcohol") will dissolve in both the gasoline and the water, causing the water to mix with the gasoline, thus moving the water from a small bubble at the bottom (where the fuel pump pickup and the carburetor jets are) into solution in the entire tank, so that it can be pulled through and burned up with the gasoline.
Ethanol will do this job as well as isopropanol, and gasoline with ethanol in it has a greater tolerance for moisture than pure gas does, BUT when you put the water into solution with the gasoline, now you have a situation where there is some water in contact with every part of your fuel system, all the time, potentially contributing to electrolysis and corrosion all over the place, instead of just in the bottom of the tank or of the float bowl.
So, putting some alcohol in your fuel system in the short term can help to dissolve water so that it can be burned and eliminated, but putting alcohol in your gas for a long time can keep water in solution and expose your entire fuel system to corrosion.
- Eric
#15
To clarify:
Ethanol (booze), Isopropanol (rubbing alcohol, and, usually "Dry Gas"), and Methanol (wood alcohol) are ALL alcohols.
ALL of them have a polar hydroxyl (OH) group on one end and a non-polar hydrocarbon (CHHH) group or groups on the other end, so all will dissolve in BOTH oil-based (nonpolar) and water-based (polar) solvents.
Methanol is the simplest: Methane with an OH group, or

Ethanol is the next-simplest: Ethane with an OH group, or

Isopropanol has 3 carbons: A propane with an OH on the middle molecule, or,
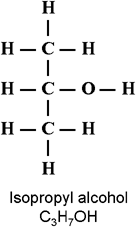
Each of these molecules has a slightly different affinity for water and for gasoline, but will dissolve in both, and, by doing that, will get both to mix together, which would not otherwise occur.
You use "Dry Gas" when you have water in your gasoline in order to get the water to mix with the gas, instead of sloshing around like a big bubble at the bottom of your tank or float bowl, and being fed to the engine, where it won't burn. The "Dry Gas" (or "alcohol") will dissolve in both the gasoline and the water, causing the water to mix with the gasoline, thus moving the water from a small bubble at the bottom (where the fuel pump pickup and the carburetor jets are) into solution in the entire tank, so that it can be pulled through and burned up with the gasoline.
Ethanol will do this job as well as isopropanol, and gasoline with ethanol in it has a greater tolerance for moisture than pure gas does, BUT when you put the water into solution with the gasoline, now you have a situation where there is some water in contact with every part of your fuel system, all the time, potentially contributing to electrolysis and corrosion all over the place, instead of just in the bottom of the tank or of the float bowl.
So, putting some alcohol in your fuel system in the short term can help to dissolve water so that it can be burned and eliminated, but putting alcohol in your gas for a long time can keep water in solution and expose your entire fuel system to corrosion.
- Eric
Ethanol (booze), Isopropanol (rubbing alcohol, and, usually "Dry Gas"), and Methanol (wood alcohol) are ALL alcohols.
ALL of them have a polar hydroxyl (OH) group on one end and a non-polar hydrocarbon (CHHH) group or groups on the other end, so all will dissolve in BOTH oil-based (nonpolar) and water-based (polar) solvents.
Methanol is the simplest: Methane with an OH group, or

Ethanol is the next-simplest: Ethane with an OH group, or

Isopropanol has 3 carbons: A propane with an OH on the middle molecule, or,
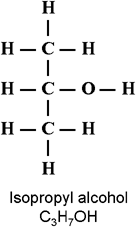
Each of these molecules has a slightly different affinity for water and for gasoline, but will dissolve in both, and, by doing that, will get both to mix together, which would not otherwise occur.
You use "Dry Gas" when you have water in your gasoline in order to get the water to mix with the gas, instead of sloshing around like a big bubble at the bottom of your tank or float bowl, and being fed to the engine, where it won't burn. The "Dry Gas" (or "alcohol") will dissolve in both the gasoline and the water, causing the water to mix with the gasoline, thus moving the water from a small bubble at the bottom (where the fuel pump pickup and the carburetor jets are) into solution in the entire tank, so that it can be pulled through and burned up with the gasoline.
Ethanol will do this job as well as isopropanol, and gasoline with ethanol in it has a greater tolerance for moisture than pure gas does, BUT when you put the water into solution with the gasoline, now you have a situation where there is some water in contact with every part of your fuel system, all the time, potentially contributing to electrolysis and corrosion all over the place, instead of just in the bottom of the tank or of the float bowl.
So, putting some alcohol in your fuel system in the short term can help to dissolve water so that it can be burned and eliminated, but putting alcohol in your gas for a long time can keep water in solution and expose your entire fuel system to corrosion.
- Eric
#16
Right here:

... Oh, and also from my brain.
And here is the MSDS for HEET: http://www.wsc.edu/facility_services...antifreeze.pdf
I lists a boiling point of 148°F and a specific gravity of 0.795.
Since the boiling point of pure methanol is 148.5°F and the specific gravity is 0.791, it would appear that this is not pure methanol, and probably contains a trace amount of water, like a couple of percent, which would not adversely affect its ability to do its job, but would make it MUCH cheaper to produce.
From the MSDS of the label, we can deduce that HEET is nearly-pure methanol. My recollection is that gas line antifreeze used to be nearly-anhydrous isopropanol, but that was years ago, and, as I noted, any hydrocarbon alcohol should work about as well as any other.
- Eric
... Oh, and also from my brain.
And here is the MSDS for HEET: http://www.wsc.edu/facility_services...antifreeze.pdf
I lists a boiling point of 148°F and a specific gravity of 0.795.
Since the boiling point of pure methanol is 148.5°F and the specific gravity is 0.791, it would appear that this is not pure methanol, and probably contains a trace amount of water, like a couple of percent, which would not adversely affect its ability to do its job, but would make it MUCH cheaper to produce.
From the MSDS of the label, we can deduce that HEET is nearly-pure methanol. My recollection is that gas line antifreeze used to be nearly-anhydrous isopropanol, but that was years ago, and, as I noted, any hydrocarbon alcohol should work about as well as any other.
- Eric
#18
Once you change the rubber connecting lines to the fuel pump and gas tank and rebuild the carb with a good kit that has rubber parts impervious to ethanol, it will run just fine. I have 3 70-72 vehicles that have run thousands of trouble free miles after making the previously mentioned changes.
#19
If you paid 79¢, then hold on to it. That'd mean you bought wood alcohol for less than $8.50 a gallon (Lowes and Home Depot sell it for $16)
A quick check of Advance, O'Reilly, and the 'Zone show that they all sell 12oz of Heet for $2.39, which is $25.50 a gallon.
- Eric
A quick check of Advance, O'Reilly, and the 'Zone show that they all sell 12oz of Heet for $2.39, which is $25.50 a gallon.
- Eric
#20
On the original point, leaded gas can still be found, all be it very rare, at some airports. Getting them to sell you some without having a plane handy can be somewhat complicated. Getting caught with it in you car's tank ... will probably mean saying g'bye to your ride.
The standard conversation in favor of lead is based around soft valve seats. Some folks don't believe it to be a real issue, some do. But a quick trip to a machine shop to install hardened valve seats renders the entire discussion moot.
I personally can attest to the value of paying a bit more per tank full of booze free super over the 10% regular unleaded. Definitely better off when you calculate dollars to miles. As for finding it, don't be shy. Don't ask the pump monkey either. He doesn't really know, or care. Ask after suppliers, and buy a testing kit. Most 91+ fuel won't have booze in it as the octane rating of the booze is too low at 89. Adding it to 87 or 89 has little effect, but it would mean having to start with higher base to keep the super octane where it needs to be.
The standard conversation in favor of lead is based around soft valve seats. Some folks don't believe it to be a real issue, some do. But a quick trip to a machine shop to install hardened valve seats renders the entire discussion moot.
I personally can attest to the value of paying a bit more per tank full of booze free super over the 10% regular unleaded. Definitely better off when you calculate dollars to miles. As for finding it, don't be shy. Don't ask the pump monkey either. He doesn't really know, or care. Ask after suppliers, and buy a testing kit. Most 91+ fuel won't have booze in it as the octane rating of the booze is too low at 89. Adding it to 87 or 89 has little effect, but it would mean having to start with higher base to keep the super octane where it needs to be.
#21
#23
Avgas is dyed like farm diesel and for much the same reason: taxes. Avgas is certainly taxed, but the wrong dept. gets the money. Having a jerry can in the trunk is fine. Having tint in the tank .. not so much.
#24
#25
Home heating oil and kerosene are dyed here, but not AvGas. My father uses it all the time in his motorcycles because it's more stable with long storage.
- Eric
#30
we run shell high test pump gas in my sons race car with no octane booster, motor has been together 4 years and race every weekend and it runs 12.30 455 motor
run the same thing in my 65 street car no problems 400 motor
run the same thing in my 65 street car no problems 400 motor
#31
That's the thing about international borders...
I think they started dyeing kerosene around here about five or six years ago.
I've still got a jug of the clear stuff.
There's a place near me that sells red-dyed #2 oil from a gas pump - when fuel prices go up, some people fill up their trucks with it. It's called "Running on Red."
- Eric
I think they started dyeing kerosene around here about five or six years ago.
I've still got a jug of the clear stuff.
There's a place near me that sells red-dyed #2 oil from a gas pump - when fuel prices go up, some people fill up their trucks with it. It's called "Running on Red."
- Eric
#32
#34
Safely? Yes.
A bit less well, if carbureted? Yes.
Greater chance of generalized fuel system corrosion? Yes.
Faster deterioration of rubber parts, especially original ones? Probably.
And I guess I didn't think of the slight blue tint of the AvGas the same way I was thinking of the inky red dye of the kerosene and #2 oil.
Do you know whether that's there for tax purposes, or, like many aviation things, for easy and unambiguous identification in order to prevent "stupid accidents"?
- Eric
A bit less well, if carbureted? Yes.
Greater chance of generalized fuel system corrosion? Yes.
Faster deterioration of rubber parts, especially original ones? Probably.
And I guess I didn't think of the slight blue tint of the AvGas the same way I was thinking of the inky red dye of the kerosene and #2 oil.
Do you know whether that's there for tax purposes, or, like many aviation things, for easy and unambiguous identification in order to prevent "stupid accidents"?
- Eric
#36
Seems gas dryer isn't a good idea for laying up over a long time, I have always filled the tank to the brim prior to long lay-ups.
I don't know if this will work now, but to remove dye from Gasoline in the UK during WW2 the trick was to filter it through bread. I think it worked for kerosene and diesel fuel too.
Roger.
I don't know if this will work now, but to remove dye from Gasoline in the UK during WW2 the trick was to filter it through bread. I think it worked for kerosene and diesel fuel too.
Roger.
#37
#38
16 bucks a gallon for methanol???? Wow I only pay 2.35 a gal for it to run in my dirt late model. And you are very right about it being corrosive to just about anything I have to replace all fuel lines every year and went to a belt drive pump because the diaphragm ones don't make it more than 8-10 nights. It even eats the carb spacer to the point it won't seal anymore after a year and turns the insides of the carb white and powdery and that's using fuel lube too.
Thread
Thread Starter
Forum
Replies
Last Post
Col Wickham
The Newbie Forum
21
August 29th, 2009 10:43 PM
Dan Wirth
General Discussion
12
August 21st, 2009 07:27 AM